

Tianjin Chemical Reagent Research Institute Google has not performed a legal analysis and makes no representation or warranty as to the accuracy of the list.) ( en Inventor 焦方喜 温莹 Current Assignee (The listed assignees may be inaccurate.
Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.) Active Application number CN 200710151072 Other languages Chinese ( zh) Google Patents CN101462949B - Method for preparing primary standard reagent potassium hydrogen phthalate
#POTASSIUM HYDROGEN PHTHALATE CODE#
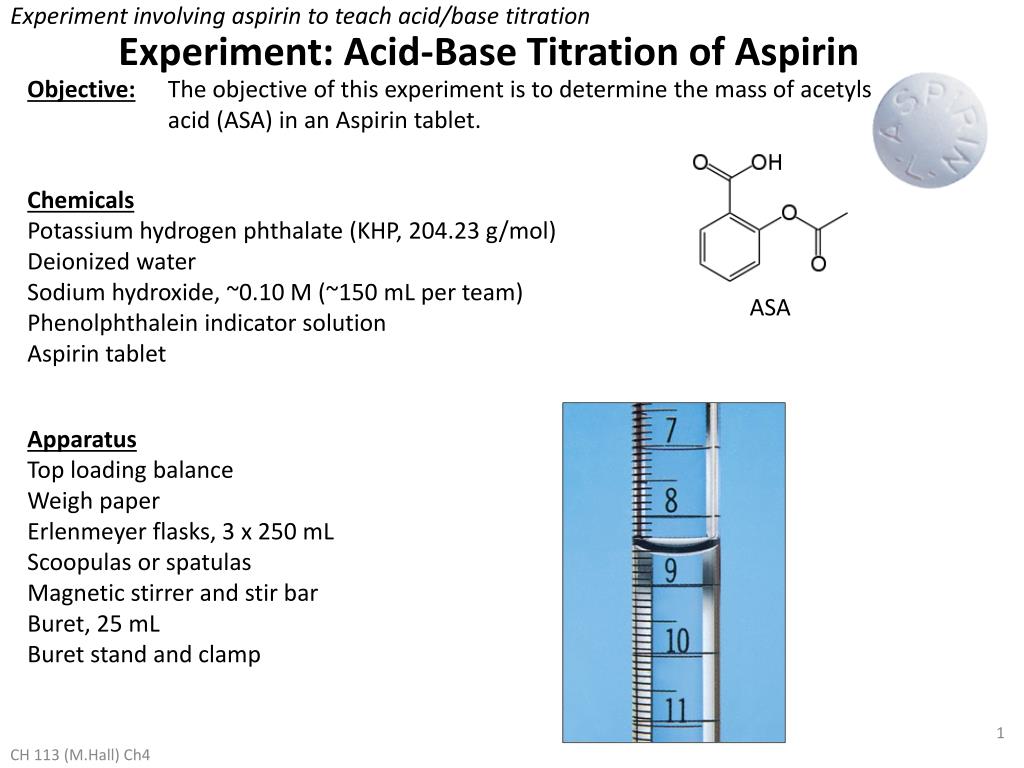
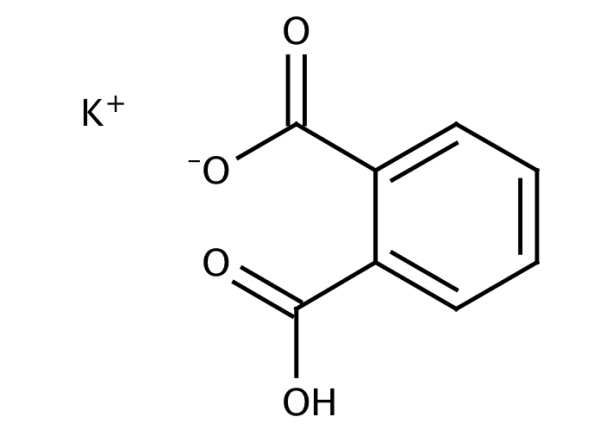
Phthalic acid, MonopotassiuM salt (8CI) potassiuM 2-carboxybenzoate PotassiuM Biphthalate, Reagent, ACS PotassiuM Hydrogen Phthalate, AcidiMetric Standard, Crystal, Reagent Special, ACS BUFFER CONCENTRATE, PH 6.00 BUFFER CONCENTRATE, PH 7.00 BUFFER MIXTURE, PH 6.8 BUFFER O BUFFER, PH 1.0 BUFFER, PH 1.00 BUFFER, PH 11.00 BUFFER, PH 1.2 BUFFER, PH 12.00 BUFFER, PH 12.45 BUFFER, PH 12.72 BUFFER, PH 1.68 BUFFER, PH 2.00 BUFFER, PH 10.00 BUFFER, PH 10.00, COLOR CODE BLUE BUFFER, PH 1.07 BUFFER PH 10, BLUE BUFFER, PH 2.5 BUFFER, PH 3.00 BUFFER SOLUTION PH 3.00 BUFFER SOLUTION PH 3.06 BUFFER SOLUTION PH 3.55 BUFFER SOLUTION PH 3.776 BUFFER SOLUTION, PH 4.0 BUFFER SOLUTION PH 4.00 BUFFER SOLUTION PH 4.006 BUFFER SOLUTION PH 6.00 BUFFER SOLUTION, PH 5.0 BUFFER SOLUTION PH 5.00 BUFFER SOLUTION, PH 5.00, +0.02 BUFFER SOLUTION PH 4.00 RED BUFFER SOLUTION, PH 4.01 BUFFER SOLUTION PH 4.65 BUFFER SOLUTION PH 4.66 BUFFER SOLUTION PH 9.180 BUFFER SOLUTION PH 9.22 BUFFER SOLUTION PH 9.23 BUFFER SOLUTION, PH 9.54 BUFFER SOLUTION, PH 8.0 BUFFER SOLUTION PH 8.00 BUFFER SOLUTION PH 8.90 BUFFER SOLUTION, PH 9.0 BUFFER SOLUTION PH 9.00 BUFFER SOLUTION PH 9.00 BLUE BUFFER SOLUTION PH 7.413 BUFFER SOLUTION PH 7.70 BUFFER SOLUTION PH 7.00 BUFFER SOLUTION PH 7.00 GREEN BUFFER SOLUTION, PH 7.2, PHOSPHATE BUFFER SOLUTION PH 6.79 BUFFER SOLUTION PH 6.86 BUFFER SOLUTION PH 6.865 BUFFER SOLUTION PH 6.88 BUFFER SOLUTION, PH 7.CN101462949B - Method for preparing primary standard reagent potassium hydrogen phthalate Before being used as a standard in volumetric analysis, analytical grade potassium hydrogen phthalate should be dried at 120o for 2hours, then allowed to cool in a desiccator.

SRM 2185_cert SRM 2185 _SDS Purification MethodsĬrystallise it first from a dilute aqueous solution of K2CO3, then H2O (3mL/g) between 100o and 0o. It finds application as a useful standard for total organic carbon testing. Potassium hydrogen phthalate is used as a primary standard for acid- base titrations as well as for calibrating pH meters. UsesĪs a primary standard for preparing volumetric alkali solutions and as a buffer in pH determinations. UsesĪs primary standard for preparing volumetric alkali solutions, also as a buffer in pH determinations. Potassium hydrogen phthalate is a certified secondary standard reference material used as a calibration buffer standard for pH instruments or pH electrodes.


 0 kommentar(er)
0 kommentar(er)
